ADmit
ADmit Therapeutics, S.L. is a company that has developed the MAP-AD® Test for the early detection of Alzheimer’s dementia and is currently in the commercialization stage.
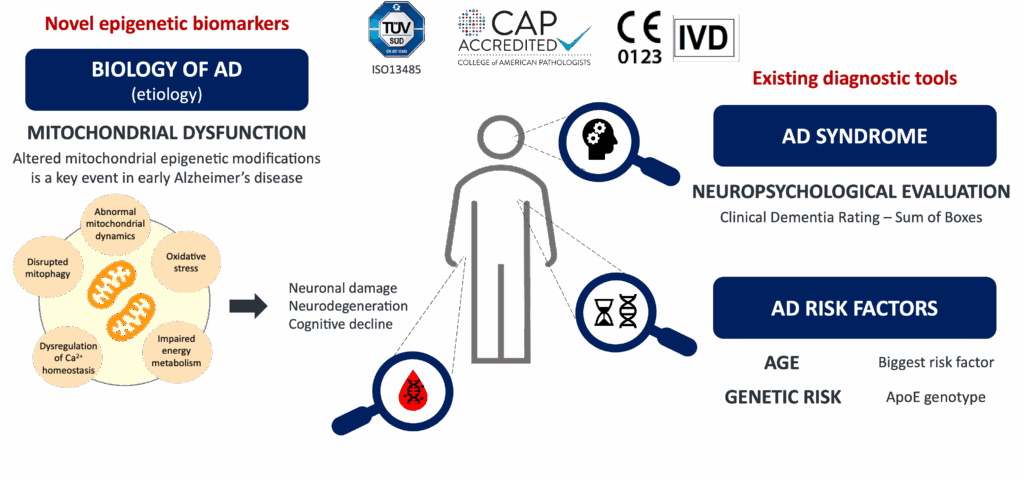
Alzheimer’s Disease (AD) is a devastating process for patients and their families and a threat to public health systems in both developed and emerging countries. It is an unmet medical need. It is estimated that more than 47 million people worldwide are suffering this disease, and that the incidence will increase considerably in the coming years due to the increase in life expectancy (130 million patients in 2050). Currently, there is an infradiagnosis of AD cases (mostly in the mild stage), and it is usually done in the moderate stage and with a delay of 12-24 months after the onset of symptoms. There is no any IVD implemented in clinical practice to predict progression to Alzheimer’s dementia in patients with Mild Cognitive Impairment.
Our IVD represents the determination of a number of biomarkers not related to either β-amyloid or tau in a blood sample. Our value proposition is based on an epigenetic analysis covering a large number of methylcytosines using next-generation sequencing techniques. Our proprietary biomarkers combined with the age, the ApoE genotype and the CDR-SOB represent a very useful tool for the prognosis of progression to Alzheimer’s dementia in Mild Cognitive Impairment patients. This cutting-edge technology provides a comprehensive analysis of the patient.
MISSION, VISION, VALUES
Our mission is to establish an early prognostic test for Alzheimer’s disease and implement it in clinical practice.
Our vision is to contribute to the identification of a curative therapy and the development of personalized medicine for Alzheimer’s disease. To this end, our prognostic test is useful for pharmaceutical companies, optimizing patient recruitment for clinical trials, facilitating the identification of new effective drugs and therapies for this devastating disease.
Our values are innovation, efficiency, effectiveness, and agility.
STRATEGY
ADmit Therapeutics provides epigenetic services to pharmaceutical companies in order to conduct clinical trials involving AD patients in the early stages of the disease, as well as to be part of the “Patient Journey” within public and private healthcare systems. The company will establish commercial and scientific agreements aimed at expanding its prognostic tools worldwide.
ADmit Therapeutics is developing a strong pipeline of projects in the field of other neurodegenerative diseases like a diagnostic IVD for the Dementia with Lewy bodies.
